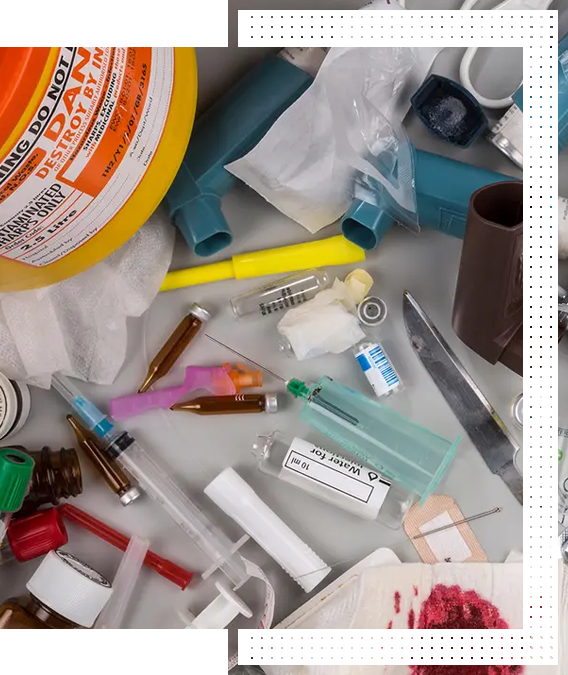
Biomedical waste (BMW) encompasses a wide range of hazardous and infectious materials generated from healthcare facilities such as hospitals, nursing homes, diagnostic laboratories, blood banks, veterinary institutions, and research facilities. Due to its potential to transmit diseases and pollute the environment, the Government of India has mandated strict guidelines under the Biomedical Waste Management Rules, 2016, amended from time to time.
As per these rules, no healthcare facility is allowed to generate, store, treat, transport, or dispose of biomedical waste without proper authorisation from the State Pollution Control Board (SPCB). This authorisation is mandatory, legally binding, and subject to periodic renewal and compliance audits.